- Home
- About Us Latest News
- Artificial Intelligence and Keratoconus - Dr Rasha Al Taie (Ophthalmologist)
Artificial Intelligence and Keratoconus - Dr Rasha Al Taie (Ophthalmologist)
Mar 31, 2021
Artificial Intelligence and Keratoconus - Dr Rasha Al Taie (Ophthalmologist)
CM Health ophthalmologist and new researcher to MMCT, Dr Rasha Al Taie, has recently been successful in receiving funding from the Potters Masonic Trust. for a study investigating the early detection of keratoconus through artificial intelligence. Keratoconus (KC) is a disease that alters the shape of the cornea (the front layer of the eye) and can lead to loss of vision, including legal blindness. New Zealand has one of the highest prevalence rates of KC worldwide, with approximately 1 in 200 adolescents identified with KC.
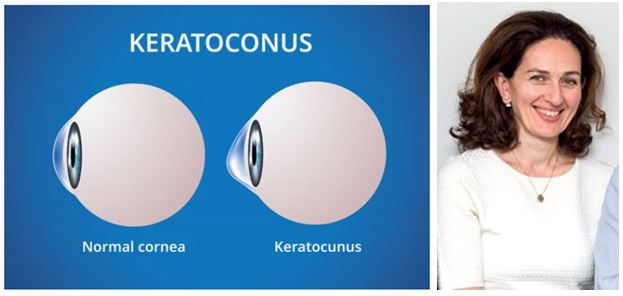
This condition disproportionately affects individuals of Māori and Pacific Island descent, with approximately 1 in 45 Māori adolescents. KC typically develops in late childhood during the teenage years, and can go undetected until there is advanced loss of vision. This is particualry true amongst individuals who are less likely to seek medical care due to cost or access difficulties. If KC is detected at an early stage there is treatment available, Corneal collagen cross linking, that can prevent progression of the disease to more advanced stages and reduce or eliminate the loss of vision. Therefore, detection of this disease at an early stage is paramount to reduce visual loss from KC.
This trial is a pilot study attempting to develop convolutional neural networks (CNN) that can form the basis of a low-cost, fast application to screen and identify KC prior to its symptomatic onset. This study started in December 2020 and will evaluate corneal topographic and tomographic images from a database of nearly 3000 patients through the Manukau Superclinic and the University of Auckland.
“My experience with the staff at Middlemore Clinical Trials has been an amazing one, especially in regards to Nicola Jackson and Renee Railton. Their knowledge and guidance in this research has been so valuable. They managed to make the whole journey enjoyable and we have learned so much from the both of them. I cannot thank them enough for all their support, advice and hard work. I think we are so lucky to have such amazing knowledgeable staff to work with. Furthermore, we would like to thank the Potters Masonic Trust for their support in funding the beginning of this research. Their support for this research is much and highly appreciated.
This is the beginning of a big project that we will continue to work on and hopefully will be able to start applying its results in clinical practices in the near future. The aim is to prevent kids from losing vision from KC in Aotearoa.”
Middlemore Clinical Trials would welcome any Opthalmology trial feasibilities from Sponsors that they wish us to consider to: feasibility@mmclintrials.nz
